Human papillomavirus (HPV) infection is the most common sexually transmitted infections globally. The link between persistent infection and cervical cancer has lead to the emergence of cancer screening programs to better stratify women and their risk of developing cervical cancer. Over 150 HPV types have been identified where some have been implicated as major risk factors in cervical cancer. The clinical significance of persistence and clearance rates of virus over time on the severity and progression, as well as co-infection of high- and low-risk HPV types, have recently been identified as factors for disease progression. However, current HPV diagnostic tools are limited in their use for the detection, identification and differentiation of multiple HPV genotypes.
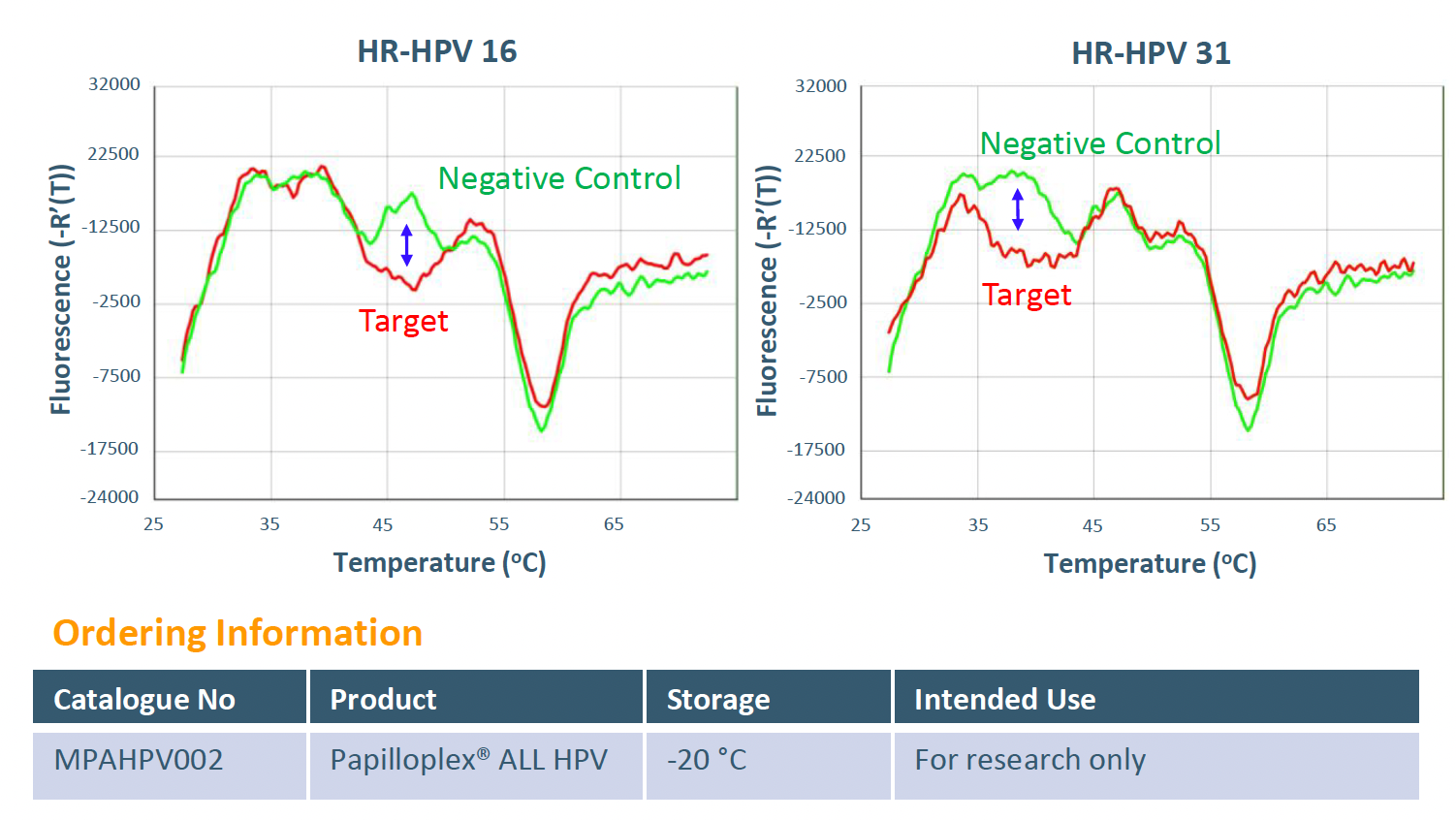
The GeneFirst Papilloplex® ALL HPV test has been designed to address these unmet medical needs by targeting 31 HPV genotypes - 15 high risk and 16 low risk - to better stratify risk for developing cervical cancers and/or sexually transmitted infections. This test, based on GeneFirst’s proprietary MPA Technology, aids our understanding of genotype-specific HPV infection in developing strategies to enhance the prevention and management of cervical cancer.
Key Features and Benefits
-
Sensitive detection and differentiation of 31 types
-
High risk: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 73
-
Low risk: 6, 11, 26, 40, 42, 43, 44, 53, 54, 61, 67, 69, 70, 72, 81, 82
-
Ease-of-use in two closed-tube reactions
-
Compatible with Applied Biosystems 7500, bio-rad CFX96 Touch, Roche LightCycler 480 and SLAN
-
Dedicated analysis software available to support data interpretation
-
Customisable, user-defined throughput