EGFR is a cell surface receptor involved in the processes of cell proliferation, adhesion and migration. It acts through initiation of several downstream signal trasnsduction cascades, mainly the Ras-Raf-MEK-ERK, PIK3 / Akt and JNK pathways. Mutations in EGFR gene have been implicated in many types of cancers. Abnormal EGFR signalling is the target of two classes of anti-EGFR drugs – monoclonal antibodies and small molecule tyrosine kinase inhibitors – both designed to block EGFR signalling. Two types of mutations can be distinguished in EGFR: activating and resistance mutations. Activating mutations increase EGFR signalling and respond to anti-EGFR treatment, resistance mutations inhibit anti-EGFR drugs efficacy and indicate poor response to anti-EGFR treatment.
EGFR-MMD-PCR Mutation Test Kit enables in four closed-tube reactions detection of 29 different somatic EGFR mutations – both activating and resistance - against a background of wild-type genomic DNA.
The GeneFirst OncoPlex® EGFR Mutation Test Kit (RUO) is a real-time PCR-based test that detects 29 different mutations with high sensitivity. This test is based on the GeneFirst proprietary technology, MMD, which is a combination of specially designed mutation-specific primers, blocker-probe and unique cycling conditions that facilitate selective amplification of multiple mutant alleles – at the same time, blocking amplification from wild type (normal) allele. This allows for the detection of rare mutations against a high background of normal, nonmutated genomic DNA.
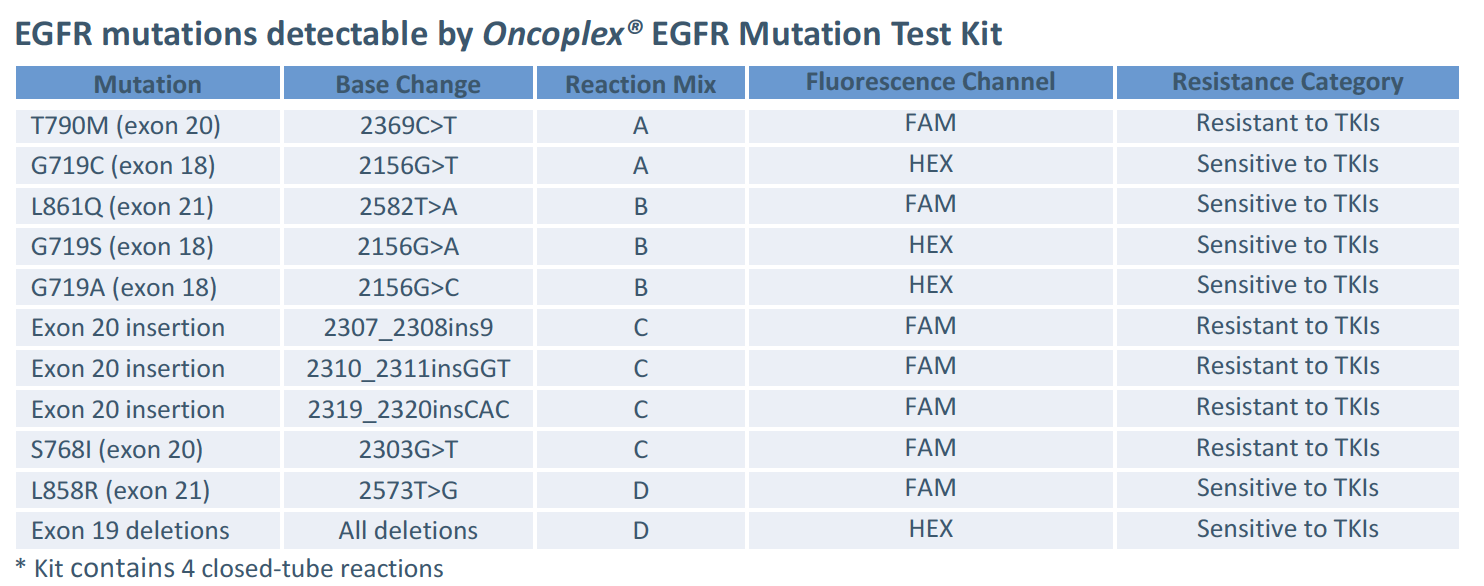
The kit allows classification of EGFR mutant variants according to their resistance / sensitivity to EGFR Tyrosine Kinase Inhibitors (TKIs). Mix A detects both T790M mutation (resistant to TKIs) and G719T mutation (sensitive to TKIs) – but can distinguish between them as they are detected in HEX and FAM channels respectively. Mixes B and C do not allow distinguishing between mutations, however mutations detected by each mix represent the same resistance category: Mix B detects EGFR mutant variants sensitive to tyrosine kinase inhibitors, Mix C – EGFR mutant variants not responsive to tyrosine kinase inhibitors. Mix D detects EGFR mutant variants sensitive to tyrosine kinase and allows distinguishing between L858R mutation (detected in FAM channel) and deletions in exon 19 (detected in HEX channel)
The EGFR-MMD-PCR Mutation Test Kit thus allows identifying patients who may benefit from cancer therapy using EGFR Tyrosine Kinase Inhibitors.